Focal Segmental Glomerulosclerosis Clinical Trial Pipeline Analysis Demonstrates 12+ Key Companies at the Horizon Expected to Transform the Treatment Paradigm, Assesses DelveInsight
Focal Segmental Glomerulosclerosis (FSGS) is a rare kidney disorder characterized by scarring (sclerosis) in the glomeruli, leading to proteinuria, progressive kidney damage, and potential kidney failure. The FSGS market is driven by rising prevalence, limited treatment options, increased recognition as an orphan disease, and ongoing development of novel targeted therapies such as endothelin receptor antagonists and anti-TGF-β agents.
New York, USA, Aug. 19, 2025 (GLOBE NEWSWIRE) -- Focal Segmental Glomerulosclerosis Clinical Trial Pipeline Analysis Demonstrates 12+ Key Companies at the Horizon Expected to Transform the Treatment Paradigm, Assesses DelveInsight
Focal Segmental Glomerulosclerosis (FSGS) is a rare kidney disorder characterized by scarring (sclerosis) in the glomeruli, leading to proteinuria, progressive kidney damage, and potential kidney failure. The FSGS market is driven by rising prevalence, limited treatment options, increased recognition as an orphan disease, and ongoing development of novel targeted therapies such as endothelin receptor antagonists and anti-TGF-β agents.
DelveInsight’s 'Focal Segmental Glomerulosclerosis Pipeline Insight 2025' report provides comprehensive global coverage of pipeline focal segmental glomerulosclerosis therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the focal segmental glomerulosclerosis pipeline domain.
Key Takeaways from the Focal Segmental Glomerulosclerosis Pipeline Report
- DelveInsight’s focal segmental glomerulosclerosis pipeline report depicts a robust space with 12+ active players working to develop 15+ pipeline focal segmental glomerulosclerosis drugs.
- Key focal segmental glomerulosclerosis companies such as Dimerix Bioscience, Travere Therapeutics, Walden Biosciences, ZyVersa Therapeutics, Sanofi, and others are evaluating new focal segmental glomerulosclerosis drugs to improve the treatment landscape.
- Promising pipeline focal segmental glomerulosclerosis therapies, such as DMX-200, Sparsentan, WAL0921, VAR 200-01, Brivekimig, and others, are in different phases of focal segmental glomerulosclerosis clinical trials.
- In June 2025, the DUPLEX trial (NCT03493685) determined that the dual endothelin angiotensin receptor antagonist sparsentan resulted in sustained proteinuria reduction among patients with focal segmental glomerulosclerosis (FSGS).
- In May 2025, Travere Therapeutics, Inc. announced that the U.S. Food and Drug Administration (FDA) had accepted its supplemental New Drug Application (sNDA) for traditional approval of FILSPARI (sparsentan) for the treatment of focal segmental glomerulosclerosis (FSGS).
- In April 2025, Dimerix Limited and Amicus Therapeutics announced that the two companies have entered into an exclusive license agreement for the commercialization of Dimerix’ Phase III drug candidate DMX-200 for all indications, including FSGS, in the United States (U.S.). Dimerix retains all rights to commercialize DMX-200 in all territories other than those already exclusively licensed.
- In January 2025, Dimerix signed an exclusive development and license agreement for the development and commercialisation of its ACTION3 Phase III drug candidate DMX-200 to treat focal segmental glomerulosclerosis (FSGS) kidney disease with Fuso Pharmaceutical Industries Pty Ltd in Japan.
Request a sample and discover the recent advances in focal segmental glomerulosclerosis drugs @ Focal Segmental Glomerulosclerosis Pipeline Report
The focal segmental glomerulosclerosis pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage focal segmental glomerulosclerosis drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the focal segmental glomerulosclerosis clinical trial landscape.
Focal Segmental Glomerulosclerosis Overview
Focal segmental glomerulosclerosis (FSGS) is a leading cause of idiopathic steroid-resistant nephrotic syndrome (SRNS) and progression to end-stage kidney disease (ESKD). The development of FSGS involves complex interactions among various glomerular cell types, including podocytes, endothelial cells, and the basement membrane. Podocytes, which are highly specialized cells, play a critical role in supporting the glomerular structure and maintaining the filtration barrier that prevents excessive protein loss in the urine. When podocytes are damaged or lost, the remaining cells enlarge to compensate, leading to foot process effacement and proteinuria. Over time, early changes such as mesangial, endothelial, and epithelial cell proliferation, along with capillary collapse or shrinkage, contribute to glomerular scarring.
Podocyte injury may arise from viral infections, toxic exposures, or intrarenal hemodynamic changes like elevated intraglomerular pressure and hyperperfusion. FSGS presents in various morphologic forms, including collapsing FSGS with mesangial hypercellularity, cellular variants with endo- and extracapillary proliferation, and forms characterized by tip lesions.
Advancements in understanding FSGS pathophysiology have identified genetic mutations in podocyte-related proteins that not only drive disease onset but also influence treatment response. For example, mutations in genes such as NPHS2 and TRPC6 often lead to poor response to immunosuppressive therapy, though patients with these mutations rarely experience disease recurrence after kidney transplantation. Additionally, variants in the APOL1 gene (G1/G2) have been linked to worse kidney outcomes and resistance to steroid treatment in FSGS and nephrotic syndrome.
Find out more about focal segmental glomerulosclerosis drugs @ Focal Segmental Glomerulosclerosis Treatment
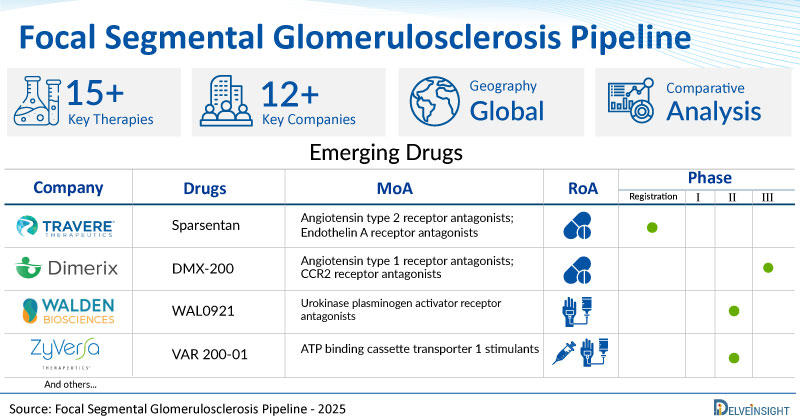
A snapshot of the Pipeline Focal Segmental Glomerulosclerosis Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Sparsentan | Travere Therapeutics | Registration | Angiotensin type 2 receptor antagonists; Endothelin A receptor antagonists | Oral |
| DMX-200 | Dimerix Bioscience | III | Angiotensin type 1 receptor antagonists; CCR2 receptor antagonists | Oral |
| WAL0921 | Walden Biosciences | II | Urokinase plasminogen activator receptor antagonists | Intravenous |
| VAR 200-01 | ZyVersa Therapeutics | II | ATP binding cassette transporter 1 stimulants | Intravenously/ Subcutaneously |
Learn more about the emerging focal segmental glomerulosclerosis therapies @ Focal Segmental Glomerulosclerosis Clinical Trials
Focal Segmental Glomerulosclerosis Therapeutics Assessment
The focal segmental glomerulosclerosis pipeline report proffers an integral view of the emerging focal segmental glomerulosclerosis therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Focal Segmental Glomerulosclerosis Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Intramuscular
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide
- Therapeutics Assessment By Mechanism of Action: Angiotensin type 2 receptor antagonists, Endothelin A receptor antagonists, Angiotensin type 1 receptor antagonists, CCR2 receptor antagonists, Urokinase plasminogen activator receptor antagonists, TRPC4 cation channel inhibitors; TRPC5 cation channel inhibitors
- Key Focal Segmental Glomerulosclerosis Companies: Dimerix Bioscience, Travere Therapeutics, Walden Biosciences, ZyVersa Therapeutics, Sanofi, and others.
- Key Focal Segmental Glomerulosclerosis Pipeline Therapies: DMX-200, Sparsentan, WAL0921, VAR 200-01, Brivekimig, and others.
Dive deep into rich insights for new focal segmental glomerulosclerosis treatments, visit @ Focal Segmental Glomerulosclerosis Drugs
Table of Contents
| 1. | Focal Segmental Glomerulosclerosis Pipeline Report Introduction |
| 2. | Focal Segmental Glomerulosclerosis Pipeline Report Executive Summary |
| 3. | Focal Segmental Glomerulosclerosis Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Focal Segmental Glomerulosclerosis Clinical Trial Therapeutics |
| 6. | Focal Segmental Glomerulosclerosis Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Focal Segmental Glomerulosclerosis Pipeline: Late-Stage Products (Phase III) |
| 8. | Focal Segmental Glomerulosclerosis Pipeline: Mid-Stage Products (Phase II) |
| 9. | Focal Segmental Glomerulosclerosis Pipeline: Early-Stage Products (Phase I) |
| 10. | Focal Segmental Glomerulosclerosis Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Focal Segmental Glomerulosclerosis Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Focal Segmental Glomerulosclerosis Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the focal segmental glomerulosclerosis pipeline therapeutics, reach out @ Focal Segmental Glomerulosclerosis Therapeutics
Related Reports
Focal Segmental Glomerulosclerosis Epidemiology Forecast
Focal Segmental Glomerulosclerosis Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted focal segmental glomerulosclerosis epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Focal Segmental Glomerulosclerosis Market
Focal Segmental Glomerulosclerosis Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key focal segmental glomerulosclerosis companies, including ACELYRIN Inc., Genentech, Inc., ChemoCentryx, Travere Therapeutics, Inc., River 3 Renal Corp., Boehringer Ingelheim, Dimerix Bioscience Pty Ltd, Chinook Therapeutics, Inc., Aldeyra Therapeutics, Inc., among others.
Chronic Kidney Disease Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key CKD companies including ProKidney, Reata Pharmaceuticals, Inc., Novo Nordisk A/S, Boehringer Ingelheim, Eli Lilly and Company, KBP Biosciences, Kibow Pharma, Cincor Pharma, AstraZeneca, Allena Pharmaceuticals, DiaMedica Therapeutics Inc., Lexicon Pharmaceuticals, Sanofi, among others.
Chronic Kidney Disease Pipeline
Chronic Kidney Disease Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key chronic kidney disease companies, including AstraZeneca, Eli Lilly and Company, Shandong Suncadia Medicine, Boehringer Ingelheim, AdAlta, Alebund Pharmaceuticals, SCOHIA PHARMA, DiaMedica Therapeutics, Roche, MC2 Therapeutics, Allena Pharmaceuticals, Regulus Therapeutics, UnicoCell Biomed, Regeneron Pharmaceuticals, among others.
Nephrotic Syndrome Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key nephrotic syndrome companies including Vertex Pharmaceuticals, Chinook Therapeutics, Novartis, Boehringer Ingelheim, Hoffmann-La Roche, Biogen, Cerium Pharmaceuticals, Otsuka Pharmaceutical Development & Commercialization, Visterra, Ionis Pharmaceuticals, Kezar Life Sciences, Alpine Immune Sciences, Dimerix Bioscience, Advanz Pharma, Apellis Pharmaceuticals, Vera Therapeutics, Inc., AstraZeneca, HI-Bio, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
